环腺苷酸对神经细胞的影响(局部使用氨甲环酸不会影响动物模型坐骨神经)
Topical Tranexamic Acid Does Not Affect Electrophysiologic or Neurovascular Sciatic Nerve Markers in an Animal Model
局部使用氨甲环酸不会影响动物模型坐骨神经的电生理或神经血管标记物的表达
Ran Schwarzkopf MD, MSc, Phuc Dang MD, Michele Luu, Tahseen Mozaffar MD, Ranjan Gupta MD
Abstract
Background Tranexamic acid is a safe and effective antifibrinolytic agent used systemically and topically to reduce blood loss and transfusion rate in patients having TKA or THA. As the hip does not have a defined capsule, topical application of tranexamic acid may entirely envelop the sciatic nerve during THA. Accidental application of tranexamic acid onto the spinal cord in spinal anesthesia has been shown to produce seizures; therefore, we sought to investigate if topical application of tranexamic acid on the sciatic nerve has a deleterious effect.
摘要
背景 氨甲环酸是一种安全有效的抗纤维蛋白溶解剂,全身和局部使用可减少TKA或THA患者的失血和输血率。由于髋关节没有明确的关节囊,全髋关节置换术中局部使用的氨甲环酸可能会完全包裹坐骨神经。腰麻中意外将氨甲环酸用于脊髓,已被证明会导致惊厥发作;因此,我们试图研究局部应用氨甲环酸是否会对坐骨神经造成伤害。
Questions/purposes We explored whether there were any short- or long-term alterations in (1) electrophysiologic measures, (2) macrophage recruitment, or (3) blood-nerve barrier permeability. Our hypothesis was that local application of tranexamic acid would have a transient effect or no effect on histologic features and function of the sciatic nerve.
问题/目的:我们探讨(1)电生理监测,(2)巨噬细胞募集,或(3)血-神经屏障通透性是否存在短期或长期改变。我们假设局部应用氨甲环酸会对坐骨神经的组织学特征和功能产生短暂影响或没有影响。
Methods We used a rat protocol to model sciatic nerve exposure in THA to determine the effects of tranexamic acid on neural histologic features and function. We evaluated 35 rats by the dorsal gluteal splitting approach to expose the sciatic nerve for topical use of control and tranexamic acid. We evaluated EMG changes (distal latency, amplitude, nerve conduction velocity), histologic signs of nerve injury via macrophage recruitment, and changes in blood-nerve barrier permeability at early (4 days) and late (1 month) times after surgery, after application of subtherapeutic (1 mg/kg body weight [1.6mg]), therapeutic (10 mg/kg [16 mg]), and supratherapeutic (100 mg/kg [160 mg]) concentrations of tranexamic acid. Differences in blood-nerve barrier permeability, macrophage recruitment, and EMG between normal and tranexamic acid-treated nerves were calculated using one-way ANOVA, with Newman-Keuls post hoc analyses, at each time. A post hoc power calculation showed that with the numbers available, we had 16% power to detect a 50% difference in EMG changes between the control, 1 mg/kg group, 10 mg/kg group, and 100 mg/kg group.
方法 采用大鼠全髋关节置换术坐骨神经暴露模型,观察氨甲环酸对坐骨神经组织学特征和功能的影响。35只大鼠,背侧臀裂入路,解剖显露坐骨神经,设局部使用氨甲环酸组和空白对照组。术后早期(4天)和晚期(1个月)进行肌电图(检测远端潜伏期、振幅、神经传导速度)、巨噬细胞募集引起的神经损伤的组织学特征以及血神经屏障通透性检查。氨甲环酸组再分三个亚组:亚治疗剂量组(1 mg/kg体重[1.6 mg])、治疗剂量组(10 mg/kg[16 mg])和超治疗剂量组(100 mg/kg[160 mg])。每次使用单因素方差分析和Newman-Keuls事后检验计算正常对照组神经和氨甲环酸处理组神经之间血神经屏障通透性、巨噬细胞募集和肌电图的差异。事后检验效能计算表明,根据现有数据,能够检验对照组、1 mg/kg组、10 mg/kg组和100mg/kg组之间肌电图变化有50%差异的效能为16%。
Results At the early and late times, with the numbers available, there were no differences in EMG except for distal latency at 4 days, macrophage recruitment, or changes in blood-nerve barrier between control rats and those with tranexamic acid-treated nerves. The distal latency in the 1 mg tranexamic acid-treated animals at 4 days was 1.06 ± 0.15 ms (p = 0.0036 versus all other groups, 95% CI, 0.89–1.25), whereas the distal latencies in the control, the 10mg/kg, and 100 mg/kg tranexamic acid treated animals were 0.83 ± 0.11, 0.89 ±0.05, and 0.87 ± 0.13, respectively. Distal latencies were not increased in any of the groups at 1 month with the numbers available (0.81 ± 0.10, 0.89 ± 0.03,0.81 ± 0.06, and 0.83 ± 0.08 ms, respectively, for controls; 1 mg/kg, 10 mg/kg, and 100 mg/kg for the tranexamic acid-treated groups).
结果 对照组与经氨甲环酸处理组在早期和晚期,除第4天的远端潜伏期、巨噬细胞募集或血神经屏障改变外,肌电图无明显差异。1 mg氨甲环酸处理组在第4天的远端潜伏期为1.06±0.15 ms(与所有其他组相比,p=0.0036,95%可信区间为0.89–1.25),而对照组、10 mg/kg和100 mg/kg氨甲环酸处理组的远端潜伏期分别为0.83±0.11、0.89±0.05和0.87±0.13。1个月时,任何一组的远端潜伏期均未增加(对照组分别为0.81±0.10、0.89±0.03、0.81±0.06和0.83±0.08ms;氨甲环酸处理组分别为1 mg/kg、10 mg/kg和100 mg/kg)。
Conclusion In our in vivo rat model study, tranexamic acid did not appear to have any clinically relevant effect on the sciatic nerve resulting from topical administration up to 1 month. However, because our statistical power was low, these data should be considered hypothesis-generating pilot data for larger, more-definitive studies.
结论 在我们活体大鼠模型研究中,局部给药1个月后,氨甲环酸对坐骨神经似乎没有任何临床相关影响。然而,由于我们的统计效能较低,这些数据应被视为产生假设的预实验数据,以便于未来进行更大规模、更明确的研究。
Clinical Relevance Topical tranexamic acid is effective in decreasing patient blood loss during THA, and results from our in vivo rat model study suggest there maybe no electrophysiologic and histologic effects on the sciatic nerve, with the numbers available, up to 1 month.
临床相关性 局部使用氨甲环酸可有效减少全髋关节置换术中患者的失血量,我们的活体大鼠模型研究结果表明,在1个月内,可能不会对坐骨神经的电生理和组织学产生影响。
Introduction
Studies have shown that tranexamic acid is an effective antifibrinolytic agent that may be used systemically in patients undergoing TKA or THA [1, 2, 4, 9, 14, 19, 26, 33, 37, 39]. Tranexamic acid (Cyklokapron1, Pfizer Inc, New York, NY, USA), a synthetic inhibitor of fibrinolysis, blocks the lysine-binding site of plasminogen and thereby serves to competitively inhibit activation of plasminogen to plasmin [3]. Studies support systemic and topical use of tranexamic acid for reducing blood loss and the need for blood transfusions without an apparent increase in the risk of deep vein thrombosis (DVT) or pulmonary embolism (PE) during arthroplasty [1,2, 8, 11, 30–32, 35–37].
前言
研究表明,氨甲环酸是一种有效的抗纤维蛋白溶解剂,可全身用于接受TKA或THA的患者[1,2,4,9,14,19,26,33,37,39]。氨甲环酸(Cyklokapron1,辉瑞公司,纽约,美国)是一种合成的抗纤维蛋白溶解剂,可阻断纤溶酶原的赖氨酸结合位点,从而竞争性抑制纤溶酶原对纤溶酶的激活[3]。研究支持全身和局部使用氨甲环酸以减少失血和输血需求,而不会明显增加关节置换术期间发生深静脉血栓形成(DVT)或肺栓塞(PE)的风险[1,2,8,11,30-32,35-37]。
Despite the reported benefits of tranexamic acid, incidences of DVT and PE have been reported after the use of tranexamic acid [18, 29]. Furthermore, accidental use of tranexamic acid in spinal anesthesia has been associated with convulsions in patients [5, 23]. Direct application of tranexamic acid in a rat spinal cord model showed it to induce seizures[30]. In addition to seizures, there are some clinical contraindications and precautions to intravenous administration of tranexamic acid, including active intravascular clotting, subarachnoid hemorrhage, previous thromboembolic event, and renal failure [34].
尽管使用氨甲环酸的益处已有报道,但也有使用氨甲环酸后发生DVT和PE的报道[18,29]。此外,腰麻中意外使用氨甲环酸后导致患者惊厥也有报道[5,23]。大鼠脊髓模型中直接使用用氨甲环酸可导致惊厥发作[30]。除惊厥发作外,静脉注射氨甲环酸还有一些临床禁忌证和预警注意征象,包括活动性血管内凝血、蛛网膜下腔出血、既往发生过血栓栓塞和肾功能衰竭[34]。
Owing to the adverse reactions associated with systemic administration of tranexamic acid, there is increasing interest in topical use of tranexamic acid to directly target the source of bleeding. With lower systemic absorption associated with topical use of tranexamic acid, there is a theoretic lower risk of thromboembolic complications, which allows for the use of tranexamic acid when systemic administration is contraindicated[35]. Although tranexamic acid has been applied topically intraarticularly in the knee during TKA and in the hip during THA with good efficacy and decreased risk of thromboembolic events [1, 15, 20, 22, 31], to our knowledge, no study to date has rigorously examined the effects of direct application of tranexamic acid on morphologic features and function of the sciatic nerve. As the hip does not have a defined capsule like the knee, topical use of tranexamic acid during THA bathes the sciatic nerve with the agent.
由于氨甲环酸全身给药相关的不良反应,人们对局部使用氨甲环酸直接作用于出血处越来越感兴趣。局部使用氨甲环酸较全身使用吸收少,理论上发生血栓栓塞并发症的风险就低,这允许在全身给药禁忌时可以局部使用氨甲环酸[35]。尽管TKA和THA时膝关节和髋关节内局部使用氨甲环酸具有良好的疗效,且血栓栓塞风险低[1,15,20,22,31],迄今为止,尚无将氨甲环酸直接作用于坐骨神经并对坐骨神经组织学形态和功能影响的研究。由于髋关节不像膝关节那样有明确的关节囊,因此在全髋关节置换术中局部使用氨甲环酸时可使坐骨神经浸泡。
We therefore investigated the effects of tranexamic acid using an in vivo animal model of the sciatic nerve. As tranexamic acid affects the clotting cascade, we explored if there were any short- or long-term alterations in (1) electrophysiologic measures, (2) macrophage recruitment, or (3) blood–nerve barrier permeability, our hypothesis being that local application of tranexamic acid would have a transient effect or no effect on histologic features and function of the sciatic nerve.
因此,使用坐骨神经活体动物模型研究氨甲环酸的作用。由于氨甲环酸影响凝血级联反应,我们探讨(1)电生理指标,(2)巨噬细胞募集,或(3)血液-神经屏障通透性是否存在短期或长期改变,假设局部应用氨甲环酸会对坐骨神经的组织学特征和功能产生短暂影响或没有影响。
Methods
To determine if tranexamic acid has any short- or long-term local and systemic effects when placed topically adjacent to the sciatic nerve, we used a rat model that simulated sciatic nerve exposure to tranexamic acid that occurs in patients undergoing THA. The sciatic nerve was examined previously for any functional nerve deficits via electrodiagnostic studies [21], histologic signs of nerve injury via macrophage recruitment [17], and changes in the blood-nerve barrier using Evans blue albumin diffusion [16].
方法
为确定坐骨神经周围局部使用氨甲环酸是否有任何短期或长期局部和全身效应,采用大鼠模型,模拟接受THA手术,暴露坐骨神经于氨甲环酸。事先进行电生理检查[21]坐骨神经是否存在任何功能性神经损害,通过巨噬细胞募集检查神经损伤的组织学表现[17],以及使用伊文思蓝白蛋白扩散检查血-神经屏障的变化[16]。
Surgical Procedure
Thirty-five male, 250-mg Sprague-Dawley rats were used in our study (Charles River Laboratories, San Diego, CA, USA). Animal surgeries were approved by the institutional Animal Care and Use Committee of the University of California, Irvine, and the study was approved by our institutional review board.
手术方法
使用35只雄性250 mgSprague-Dawley大鼠(美国加利福尼亚州圣地亚哥查尔斯河实验室)。动物手术已获加州大学欧文分校动物保护和使用委员会的批准,该研究已获本单位审查委员会的批准。
All rats were anesthetized with an intraperitoneal injection of ketamine and xylazine at 80 to 100 mg/kg and 5 to 10 mg/kg [17]. A dorsal gluteal-splitting approach was used on both rat hind limbs to directly expose the sciatic nerves in each animal, as would be performed during routine THA. One milliliter of normal saline solution containing three different concentrations (subtherapeutic, 1 mg/kg; therapeutic, 10 mg/kg; or supratherapeutic, 100 mg/kg) of tranexamic acid was placed topically in the right hind leg near the sciatic nerve. Ten milligrams per kilogram was selected as a therapeutic concentration as those used in TKA and THA range from 500 mg to 3 g for an adult human whose average weight is approximately 70 kg to 85 kg [12,20]. One milliliter of normal saline control was placed in the left hind leg near the sciatic nerve as a matched control. To limit variability, all surgical procedures were performed by the senior author (RG). None of the study rats died from our initial surgery and all remained ambulatory without any gross deficit. All rats completed the study protocol.
所有大鼠均以腹腔注射氯胺酮(80-100 mg/kg)和甲苯噻嗪(5-10 mg/kg)进行麻醉[17]。大鼠两条后肢使用背侧臀裂入路,像常规THA那样直接暴露每只动物的坐骨神经,将三种不同浓度(亚治疗组,1 mg/kg;治疗组,10 mg/kg;或超治疗组,100 mg/kg)的1毫升氨甲环酸氯化钠溶液局部置于右后腿坐骨神经附近。选择10mg/kg作为治疗浓度,是因为TKA和THA中使用的浓度,对于平均体重约为70kg至85kg成年人,剂量范围为0.5-3g[12,20]。左后腿坐骨神经附近注入1毫升生理盐水作为对照组。为减少手术偏倚,所有手术均由资深作者(RG)完成。整个研究中没有一只大鼠死于手术,所有大鼠都可行走,没有任何严重缺陷。所有大鼠均完成研究方案。
Animals were euthanized by an anesthetic overdose [17]. For characterization of the blood-nerve barrier integrity and macrophage recruitment, animals were sacrificed at two different times after tranexamic acid application: 4 days (n = 5, 1 mg/kg; n = 5, 10 mg/kg; n = 5, 100 mg/kg; and n = 3, no tranexamic acid as negative controls); and 1 month (n = 5, 1mg/kg; n = 5, 10 mg/kg; n = 5, 100 mg/kg; and n = 2, no tranexamic acid as negative controls). Experimental (right sciatic) and saline-injected control (left sciatic) nerves were harvested from each animal.
用过量麻醉剂安乐死动物[17]。为保证血神经屏障完整性和巨噬细胞募集,氨甲环酸处理后两个不同时间点处死动物:4天(n=5, 1 mg/kg;n=5,10 mg/kg;n=5, 100 mg/kg;n=3,无氨甲环酸作为阴性对照);1个月(n=5, 1 mg/kg;n=5,10 mg/kg;n=5, 100 mg/kg;n=2,无氨甲环酸作为阴性对照)。采集实验组右侧坐骨神经和对照组左侧坐骨神经。
EMG
Before animal sacrifice, EMG was performed in vivo on all animals by a qualified board-certified neurologist (TM), to determine distal latency, amplitude, and nerve conduction velocity [10]. Motor conduction studies in the sciatic-tibial fibers were performed by stimulating the sciatic nerve at the sciatic notch and popliteal region using a monopolar needle electrode. The reference needle-stimulating electrode was placed in the ipsilateral lumbar paraspinal muscle. The M-wave (compound motor action potential) from the peroneal-innervated ankle dorsiflexor muscle (tibialis anterior) was recorded by placing a subdermal electroencephalogram needle electrode in the muscle approximately 3 mm above the ankle. The distal latency, amplitude of the response, and nerve conduction velocity were computed. The reference recording electrode was inserted in the plantar aspect of the foot. All neurophysiologic recordings were obtained using a Cadwell Sierra® LT machine (Cadwell Laboratories, Kennewick, WA, USA).
肌电图检查
处死动物前,由有资质的专业神经学家(TM)对所有动物进行肌电图检查,以确定远端潜伏期、振幅和神经传导速度[10]。通过单电极针刺激坐骨切迹和腘区的坐骨神经,检测坐骨神经-胫神经纤维的运动传导。参考电极刺激针放置在同侧腰椎椎旁肌。将皮下脑电图针状电极置于踝关节上方约3mm处的肌肉中,记录腓总神经支配的踝关节背屈肌(胫前肌)的M波(复合运动动作电位)。计算远端潜伏期、反应幅度和神经传导速度。参考电极插入足底。所有神经电生理记录均使用Cadwell Sierra® LT仪器(美国华盛顿州肯纳维克Cadwell实验室)获得。
Tissue Processing
After harvest, sciatic nerves were preserved in 4% paraformaldehyde solution at 4℃ overnight and then cryoprotected in serial dilutions of sucrose for 3 hours each (10%, 20%, and 30% sucrose in 0.1 mol/L phosphate buffered saline [PBS]). Nerve samples were frozen, embedded in a 1:1 mixture of optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and Aqua Mount® (Lerner Laboratories, Pittsburgh, PA, USA). Specimens were cut in 15-μm-thick cross sections with a cryostat, mounted on poly-L-lysine-coated glass slides (Fisher Scientific, Pittsburgh,PA, USA), and stored at -80℃ until testing.
标本制备
切取坐骨神经标本,4%多聚甲醛溶液4℃保存过夜,然后在阶梯浓度蔗糖中冷冻保护各3小时(10%、20%和30%蔗糖,用0.1 mol/L磷酸盐缓冲液[PBS]配制)。冷冻,用最佳切割温度化合物(美国加利福尼亚州托伦斯市SakuraFinetek)和Aqua Mount®(美国宾夕法尼亚州匹兹堡勒纳实验室)的1:1混合物包埋。用低温恒温器将标本横切成15 μm厚的切片,置于聚赖氨酸涂层玻片(Fisher Scientific,匹兹堡,宾夕法尼亚州,美国),-80℃下储存至检测。
Immunohistochemistry
Immunohistochemistry was performed on the sciatic nerve cross sections to detect ED1-IR, a marker specific for activated macrophages [17]. Positive control samples of the spleen and liver were used to detail localization of hematogenously derived macrophages. Frozen sections of the nerve, spleen, and liver were fixed in paraformaldehyde and immersed in 0.1% Triton® X-100 in PBS (Thermo Fisher Scientific, NJ, USA). Nonspecific binding was blocked with 10% goat antibodies or bovine serum albumin. Sections were incubated with mouse anti-rat ED1-IR (1:300; Chemicon, Temecula, CA, USA) overnight at 4 ℃. Control sections were incubated without exposure to primary antibodies. After thorough washes with PBS, slides were incubated in fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin (IgG, 1:500; Chemicon)for 1 hour. After washes in PBS, slides were counterstained and mounted with Vectashield® antifade solution containing 40,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) and viewed with an Olympus fluorescent microscope (Olympus Corporation, Center Valley, PA, USA) equipped with PathVysion® software (Applied Imaging, San Jose, CA, USA).
免疫组织化学检查
坐骨神经横截面切片进行免疫组织化学染色以检测活化巨噬细胞特异性标记物ED1-IR [17]。用脾脏和肝脏的阳性对照标本,定位和呈现血源性巨噬细胞。用多聚甲醛固定神经、脾脏和肝脏冰冻切片,浸入PBS-0.1%Triton®X-100溶液(美国新泽西州赛默飞世尔科学公司)。用10%山羊抗体或小牛血清白蛋白阻断非特异性结合。4℃切片与小鼠抗鼠ED1-IR(1:300;Chemicon,Temecula,CA,USA)孵育过夜。对照组未加抗体孵育。PBS彻底冲洗,载玻片用异硫氰酸荧光素标记的山羊抗小鼠免疫球蛋白(IgG,1:500;Chemicon)培养1小时。PBS洗涤,用含40,6-二氨基-2-苯基吲哚的Vectashield®防褪色溶液(DAPI;Vector Laboratories,Burlingame,CA,USA)对载玻片进行复染和固定,用配有PathVysion®软件(Applied Imaging,San Jose,CA,USA)的奥林巴斯荧光显微镜(美国宾夕法尼亚州中心谷奥林巴斯公司)读片。
Immunohistochemical analysis was performed to identify endothelial cells lining the lumen of microvessels in the nerve sections. Frozen sections were immersed in 4% paraformaldehyde and then blocked with 10% normal goat serum and 0.1% Triton® X-100 in PBS for 1 hour. The sections were incubated overnight at 4℃ with anti-rat endothelial cell antigen-1 (anti-RECA 1; 1:200; Serotec, Bicester, UK). After washes with PBS, the sections were incubated in fluorescein-conjugated goat anti-mouse IgG (1:200) for 1 hour. Slides were washed with PBS, stained with DAPI, and observed with an Olympus IX-71 fluorescence microscope.
免疫组织化学分析以确定神经切片中微血管管腔内的内皮细胞。4%多聚甲醛浸泡冰冻切片,然后用10%正常山羊血清和0.1% Triton® X-100 PBS溶液封闭1小时。切片在4℃下与抗大鼠内皮细胞抗原-1(抗RECA 1;1:200;英国BicesterSerotec)孵育过夜。PBS洗涤,切片与荧光素结合山羊抗鼠IgG(1:200)培养1小时。PBS洗涤,DAPI染色,并用Olympus IX-71荧光显微镜观察。
Macrophage Recruitment
Using an Olympus IX-71 microscope equipped with SlideBookTM 4.1 (Intelligent Imaging Innovations, Denver, CO, USA), the total numbers of ED1-IR stained cells were counted on specimens harvested at 4 days and 1 month after surgery. Only labeled cells with visible nuclei were counted. Macrophage numbers were counted under 40 X per 100 μm X 100μm section. For each nerve, multiple sections were prepared. Three to five sections were selected for analysis, each with intact axonal structural integrity. A 100μm X 100μm section then was selected randomly to account for variability in and between specimens.
巨噬细胞募集
使用配有SlideBookTM 4.1(美国科罗拉多州丹佛市智能成像创新公司)的Olympus IX-71显微镜,计算术后4天和1个月标本上ED1-IR染色细胞的总数。40倍放大,100μm X 100μm为单个面积单位,计算可见细胞核的巨噬细胞数量。对每条神经,准备多个切片。选择3-5个切片进行分析,每个切片都具有完整的轴突结构。随机选择一个100μm X100μm截面面积,以降低样品间的偏倚。
Evans Blue Albumin
Blood-nerve barrier permeability (integrity) was evaluated using Evans Blue albumin, a solution of 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) mixed with 1% Evans Blue dye (Sigma-Aldrich) in sterile distilled water and filtered through a G-25 Medium Sephadex® column (Sigma-Aldrich, St Louis, MO, USA). In accordance with previously described methods [16, 24], 1 mL Evans Blue albumin per 100 g body weight was injected intravenously, under anesthesia, and allowed to circulate for 15 minutes before the sciatic nerves were harvested and prepared as described. Before evaluation, nerve cross sections were counterstained with DAPI to localize cell nuclei.
伊文思蓝白蛋白染色
用5%牛血清白蛋白(美国密苏里州圣路易斯市西格玛奥尔德里奇)与1%伊文思蓝染料(美国密苏里州圣路易斯市西格玛奥尔德里奇)加无菌蒸馏水,制备伊文思蓝白蛋白,评估血神经屏障通透性(完整性)。通过G-25Medium Sephadex®柱(美国密苏里州圣路易斯市西格玛奥尔德里奇)过滤。根据之前描述的方法[16,24],麻醉下静脉注射1 mL/100g体重伊文思蓝白蛋白,循环15分钟后,按照前述方法采集和制备坐骨神经标本。用DAPI对神经横截面进行复染以定位细胞核后进行分析。
Quantification of Changes in Blood-nerve Barrier Permeability
As reported [16], functionality of the blood-nerve barrier was evaluated by comparing the fluorescence of the Evans Blue albumin in the neural microvasculature with surrounding endoneurium. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to determine the average optical density of five random 10X10 pixel areas (approximately 4 μm2) in a randomly selected microvessel and five random 10X10 pixel areas from the surrounding endoneurium. The comparative ratio of intensity in a blood vessel to endoneurium was calculated by dividing the average intensity of the five blood vessel optical density measurements by the average optical density of the five endoneurial measurements (Fig. 1).
量化血神经屏障通透性
如文献[16]所述,通过比较神经微血管和周围神经内膜中伊文思蓝白蛋白的荧光以评估血神经屏障功能。用ImageJ(美国马里兰州贝塞斯达国立卫生研究院)测定随机选取的五个10X10像素微血管区域(约4 μm2)和五个10X10像素周围神经内膜区域的平均光密度。将五个微血管区域光密度平均值除以五个神经内膜区域光密度平均值,计算血管与神经内膜光密度比率(图1)。

Fig. 1 An image of a blood vessel in a sciatic nerve axon shows the method of quantification of changes in blood-nerve barrier permeability. Extravasation of Evans Blue albumin is red, cell nuclei stained with DAPI are blue; anti-RECA-1 for endothelial cells is green; the black area inside the green endothelial cell layer is the lumen of a vessel, and the black area outside the green is the extracellular matrix. With ImageJ, the optical density intensity was measured by selecting five random areas in a microvessel (white squares) and five random areas from the surrounding endoneurium (yellow squares). All squares represent 10X10 pixel areas (approximately 4 μm2).
图1. 坐骨神经轴突中的血管图像量化血神经屏障通透性变化的方法。伊文思蓝白蛋白外渗为红色,DAPI染色的细胞核为蓝色;抗RECA-1的内皮细胞为绿色;绿色内皮细胞层内的黑色区域为血管腔,绿色内皮细胞层外的黑色区域为细胞外基质。用ImageJ随机选取微血管五个区域(白色方块)和周围神经内膜的五个区域(黄色方块)来测量光密度强度。所有正方形代表10X10像素区域(约4μm2)。
Statistical Analysis
All statistical analyses were performed using Prism 5 (GraphPad, San Diego, CA, USA). Data are presented as the mean and SD. Differences in Evans Blue albumin optical density, macrophage recruitment (ED1-IR cells), and EMG (distal latency, amplitude, nerve conduction velocity) between normal and tranexamic acid-treated nerves were calculated using one-way ANOVA, with Newman-Keuls post hoc analyses, at each time. A post hoc power calculation showed that with the numbers available, we had 16% power to detect a 50% difference in EMG changes between the control group, 1 mg/kg group, 10 mg/kg group, and 100 mg/kg group.
统计分析
所有统计分析均使用Prism 5(美国加利福尼亚州圣地亚哥GraphPad)进行。数据以平均值和标准差表示。每次使用单因素方差分析和Newman-Keuls事后检验计算正常对照神经和氨甲环酸处理神经之间伊文思蓝白蛋白光密度、巨噬细胞募集(ED1-IR细胞)和EMG(远端潜伏期、振幅、神经传导速度)的差异。事后效能计算表明,根据现有数据,我们有16%的效能能够检验对照组、1 mg/kg组、10 mg/kg组和100 mg/kg组之间肌电图变化的50%差异。
Results
At 4 days (Fig. 2) and at 1 month (Fig. 3) after surgery, there were no differences between the control group nerves nor any of the treatment groups in terms of nerve conduction velocity or amplitude, with the numbers available (Table 1). There was an increase in distal latency in the 1 mg/kg tranexamic acid-treated group only at 4 days, but the difference did not persist at 1 month, with the numbers available. The distal latency in the 1 mg tranexamic acid treated animals at 4 days was 1.06 ± 0.15 ms (p =0.0036 versus all other groups), whereas the distal latencies in the control, the 10 mg/kg, and 100 mg/kg tranexamic acid treated animals were 0.83 ± 0.11, 0.89 ± 0.05, and 0.87 ± 0.13 ms, respectively. Distal latencies were not increased in any of the groups at 1 month with the numbers available (0.81 ± 0.10, 0.89 ±0.03, 0.81 ± 0.06, and 0.83 ± 0.08 ms, respectively, for controls; 1 mg/kg, 10mg/kg, and 100 mg/kg for the tranexamic acid-treated groups) (Table 2).
结果
术后4天(图2)和1个月(图3)时,根据现有数据,对照组和各实验组的神经传导速度或波幅均无差异(表1)。1 mg/kg氨甲环酸处理组的远端潜伏期仅在术后4天时增加,但根据现有数据,但1个月后不再有差异。1 mg氨甲环酸处理组动物在术后第4天的远端潜伏期为1.06±0.15 ms(与所有其他组相比p=0.0036),而对照组、10 mg/kg和100 mg/kg氨甲环酸处理组动物的远端潜伏期分别为0.83±0.11、0.89±0.05和0.87±0.13ms。在术后1个月时,任何一组的远端潜伏期均未增加(对照组分别为0.81±0.10、0.89±0.03、0.81±0.06和0.83±0.08 ms;氨甲环酸处理组分别为1 mg/kg、10 mg/kg和100 mg/kg)(表2)。



Fig. 2A–C The graphs show (A) distal latency, (B) amplitude, and (C) nerve conduction velocity of a rat sciatic nerve at 4 days. The control group (n = 18) had the sciatic nerve exposed to saline; the 1 mg/kg group (n = 5) had the sciatic nerve exposed to 1 mg/kg tranexamic acid; the 10 mg/kg group (n = 5) had the sciatic nerve exposed to 10 mg/kg tranexamic acid; and the 100 mg/kg group (n =5) had the sciatic nerve exposed to 100 mg/kg tranexamic acid; *Significant (p= 0.0036).
图2A-C. 显示大鼠坐骨神经在术后4天的(A)远端潜伏期,(B)波幅和(C)神经传导速度。对照组(n=18)坐骨神经暴露于盐水中;1mg/kg组(n=5)坐骨神经暴露于1mg/kg氨甲环酸;10mg/kg组(n=5)坐骨神经暴露于10mg/kg氨甲环酸;100mg/kg组(n=5)坐骨神经暴露于100mg/kg氨甲环酸。*有统计学差异(p=0.0036)。
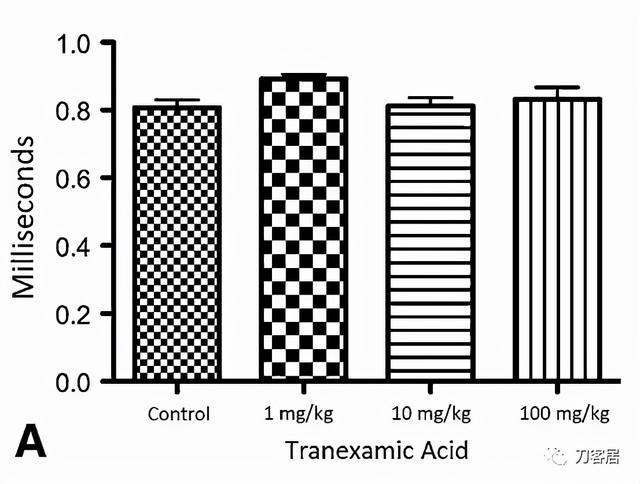
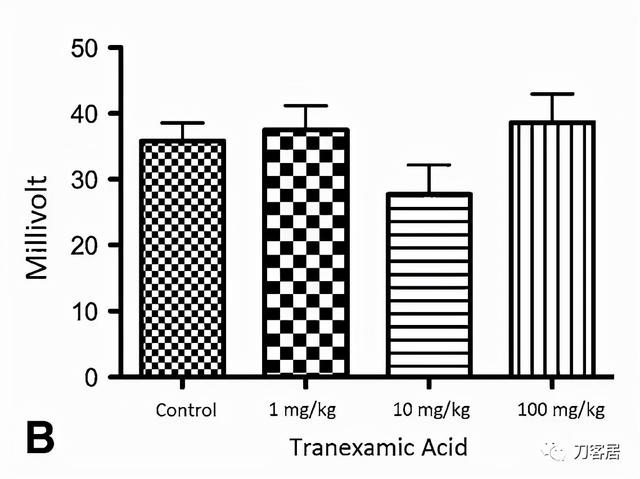

Fig. 3A–C The graphs show (A) distal latency, (B) amplitude, and (C) nerve conduction velocity of rat sciatic nerve at 1 month. Control rats (n = 17) were those with sciatic nerves exposed to saline. The 1 mg/kg are rats (n = 5) whose sciatic nerve was exposed to 1 mg/kg of tranexamic acid. The 10 mg/kg are rats (n = 5) whose sciatic nerve was exposed to 10 mg/kg of tranexamic acid. The 100 mg/kg are rats (n = 5) whose sciatic nerve was exposed to 100 mg/kg of tranexamic acid; p = 0.38, which did not reach significance*.
图3A-C. 显示大鼠坐骨神经术后1个月的(A)远端潜伏期,(B)波幅和(C)神经传导速度。对照组(n=17)为坐骨神经暴露于盐水中。1 mg/kg组(n=5)为坐骨神经暴露于1 mg/kg氨甲环酸。10mg/kg组(n=5)为坐骨神经暴露于10mg/kg氨甲环酸。100mg/kg组(n=5)为坐骨神经暴露于100mg/kg氨甲环酸;p=0.38。无统计学差异*。

表1. 术后4天对照组及实验组电生理检查结果

表2. 术后1月对照组及实验组电生理检查结果
There were no differences when quantifying the amount of macrophages between treated and control rats with the numbers available. Four days after surgery, 2.6 ± 1.27 macrophages were seen in the control sciatic nerve sections, 3.2 ± 1.10 macrophages in the 1 mg/kg tranexamic acid-treated nerve sections, 1.8 ± 0.84 macrophages in the 10 mg/kg tranexamic acid-treated nerve sections, and 1.8 ± 0.84 macrophages in the 100 mg/kg tranexamic acid-treated nerve sections (p = 0.14) (Fig. 4). At 1 month after surgery, 2.5 ± 0.87 macrophages were seen in the control sciatic nerve sections, 2.2 ± 0.84 macrophages in the 1 mg/kg tranexamic acid-treated nerve sections, 2.2 ± 0.84 macrophages in the 10 mg/kg tranexamic acid treated nerve sections, and 1.4 ± 0.55 macrophages in the 100 mg/kg tranexamic acid-treated nerve sections (p = 0.09) (Fig. 5).
根据现有数据,实验组和对照组大鼠之间的巨噬细胞数量量化没有差异。术后4天,对照组坐骨神经切片可观察到2.6±1.27个巨噬细胞,1 mg/kg氨甲环酸处理组神经切片可观察到3.2±1.10个巨噬细胞,10 mg/kg氨甲环酸处理组神经切片可观察到1.8±0.84个巨噬细胞,100 mg/kg氨甲环酸处理组神经切片中可观察到1.8±0.84个巨噬细胞(p=0.14)(图4)。术后1个月,对照组坐骨神经切片可观察到2.5±0.87个巨噬细胞,1 mg/kg氨甲环酸处理组神经切片可观察到2.2±0.84个巨噬细胞,10 mg/kg氨甲环酸处理组神经切片中可观察到2.2±0.84个巨噬细胞,100 mg/kg氨甲环酸处理组神经切片中可观察到1.4±0.55个巨噬细胞(p=0.09)(图5)。

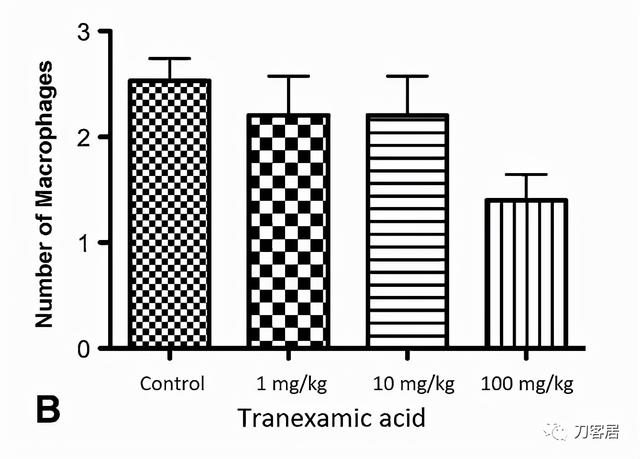
Fig. 4A–B Macrophage recruitment quantifications at (A) 4 days and (B) 1 month using ED1-IR marker for macrophages are shown. Control rats (n = 17) were those whose sciatic nerve was exposed to saline; 1 mg/kg rats (n = 5) had the sciatic nerve exposed to 1 mg/kg tranexamic acid; 10 mg/kg rats (n = 5) had the sciatic nerve exposed to 10 mg/kg tranexamic acid, and 100 mg/kg rats (n = 5) had the sciatic nerve exposed to 100 mg/kg tranexamic acid. *Significant.
图4A-B. 显示术后4天(A)和术后1个月(B)时使用ED1-IR标记物定量巨噬细胞募集。对照组(n=17)为坐骨神经暴露于盐水;1mg/kg组(n=5)坐骨神经暴露于1mg/kg氨甲环酸;10mg/kg组(n=5)坐骨神经暴露于10mg/kg氨甲环酸,100mg/kg组(n=5)坐骨神经暴露于100mg/kg氨甲环酸。*有统计学差异。


Fig. 5A–B The blood-nerve barrier integrity examinations for extravasation using Evans Blue albumin at (A) 4 days and at (B) 1 month are shown. Control rats (n = 17) were those whose sciatic nerve was exposed to saline; 1 mg/kg rats (n = 5) had the sciatic nerve exposed to 1 mg/kg tranexamic acid; 10 mg/kg rats (n = 5) had the sciatic nerve exposed to 10 mg/kg tranexamic acid, and 100 mg/kg rats (n = 5) had the sciatic nerve exposed to 100 mg/kg tranexamic acid. *Significant.
图5A-B. 显示术后4天(A)和术后1个月(B)时使用伊文思蓝白蛋白进行外渗血神经屏障完整性检测。对照组(n=17)为坐骨神经暴露于盐水;1mg/kg组(n=5)为坐骨神经暴露于1mg/kg氨甲环酸;10mg/kg组(n=5)为坐骨神经暴露于10mg/kg氨甲环酸,100mg/kg组(n=5)为坐骨神经暴露于100mg/kg氨甲环酸。*有统计学差异。
No differences in leakage of Evans Blue albumin (blood-nerve barrier permeability or integrity) were observed in the control (saline) samples, in the 1 mg/kg tranexamic acid, 10 mg/kg tranexamic acid, and 100 mg/kg tranexamic acid-treated nerves at 4 days or 1 month (Fig.6) after surgery with the numbers available. At 4 days, the ratio of Evans Blue albumin leakage was 2.40 ± 0.96 for controls, 3.67 ± 1.27 for the 1 mg/kg group, 3.92 ± 2.21 for the 10 mg/kg group, and 3.14 ± 1.10 for the 100 mg/kg group (p =0.10). At 1 month, the ratio of leakage was 2.52 ± 0.80 for the control group, 1.78 ± 0.22 for the 1 mg/kg group, 2.78 ± 0.76 for the 10 mg/kg group, and 2.34± 0.60 for the 100 mg/kg group (p = 0.15).
根据现有数据,术后4天或1个月(图6),对照组(生理盐水处理)、1 mg/kg氨甲环酸、10 mg/kg氨甲环酸和100 mg/kg氨甲环酸处理组的坐骨神经中伊文思蓝白蛋白渗漏(血神经屏障通透性或完整性)无统计学差异。术后第4天,对照组伊文思蓝白蛋白渗漏率为2.40±0.96,1 mg/kg组为3.67±1.27,10mg/kg组为3.92±2.21,100 mg/kg组为3.14±1.10(p=0.10)。术后1个月时,对照组渗漏率为2.52±0.80,1mg/kg组为1.78±0.22,10mg/kg组为2.78±0.76,100mg/kg组为2.34±0.60(p=0.15)。
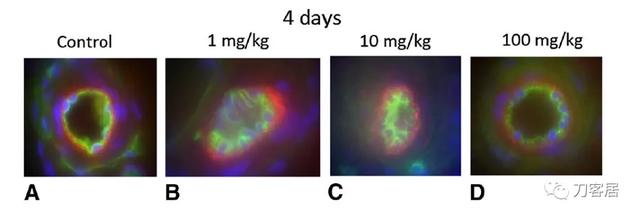

Fig. 6A–H The blood-nerve barrier integrity examinations using Evans Blue albumin show extravasation (red) at 4 days for the (A) control group, and (B) 1 mg/kg, (C) 10 mg/kg, and (D) 100 mg/kg groups. The examinations also are shown for (E) the control group, and (F) 1 mg/kg, (G) 10 mg/kg, and (H) 100 mg/kg groups at 1 month. Blue is stained with DAPI for cell nuclei. Green is stained with ED1-IR for endothelium.The black area inside the green endothelium is the lumen of a vessel. The black area outside the green is the extracellular matrix. The control rats (n = 17) had the sciatic nerve exposed to saline, the 1 mg/kg rats (n = 5) had the sciatic nerve exposed to 1 mg/kg tranexamic acid, the 10 mg/kg rats (n = 5) had the sciatic nerve exposed to 10 mg/kg tranexamic acid, and the 100 mg/kg rats (n= 5) had the sciatic nerve exposed to 100 mg/kg tranexamic acid.
图6A-H.对照组(A)和1 mg/kg组(B),10 mg/kg组(C)和100mg/kg组(D)术后第4天,伊文思蓝白蛋白检测血神经屏障完整性(红色)。术后1个月,对照组(E),1 mg/kg组(F),10 mg/kg组(G)和100 mg/kg组(H)血神经屏障完整性的检测结果。DAPI对细胞核进行蓝色染色。内皮细胞ED1-IR染色呈绿色。绿色内皮内侧黑色区域为血管管腔。绿色外的黑色区域为细胞外基质。对照组(n=17)坐骨神经暴露于盐水,1mg/kg组(n=5)坐骨神经暴露于1mg/kg氨甲环酸,10mg/kg组(n=5)坐骨神经暴露于10mg/kg氨甲环酸,100mg/kg组(n=5)坐骨神经暴露于100mg/kg氨甲环酸。
Discussion
Numerous reports suggest that use of intravenous tranexamic acid is effective in reducing patient blood loss without increasing the risk of thromboembolic events in select cases of total joint arthroplasty [6, 7, 31–33, 37]. However, safety concerns have been raised [18, 29] pertaining to thromboembolic, cardiovascular, and neurologic risks with the use of tranexamic acid. In particular, seizures have been reported when tranexamic acid is accidently placed onto the spinal cord during spinal anesthesia and when directly applied to the rat spinal cord [5, 23, 30]. We therefore sought to determine whether use of tranexamic acid has an effect on histologic features and function of the sciatic nerve by evaluating EMG changes (distal latency, amplitude, nerve conduction velocity), histologic signs of nerve injury via macrophage recruitment, and changes in blood nerve barrier permeability at early (4 days) and late (1 month) times after surgery. We found that at both times, topical application of tranexamic acid in a rat model did not appear to have any effect on histologic features and function of the sciatic nerve, with the numbers available.
大量研究表明,部分全关节置换手术病例中,静脉注射氨甲环酸可有效减少出血,且不会增加血栓栓塞风险[6,7,31–33,37]。然而,使用氨甲环酸有可能引起血栓栓塞、心血管和神经损伤的安全问题,已有关注[18,29]。特别是,当氨甲环酸在腰麻时意外直接作用于脊髓以及在大鼠实验时将氨甲环酸直接应用于脊髓时,有报道称可导致惊厥发作[5,23,30]。因此,我们试图通过肌电图(远端潜伏期、波幅、神经传导速度)、巨噬细胞募集对正常及氨甲环酸处理的坐骨神经组织学特征进行研究,以图了解氨甲环酸是否对坐骨神经的组织学特征和功能产生影响,以及术后早期(4天)和晚期(1个月)血神经屏障通透性的变化。结果发现,在这两个时间点,根据现有数据,大鼠模型中局部应用氨甲环酸似乎对坐骨神经的组织学特征和功能没有任何影响。
Limitations of our study include the small number of animals, non-randomization of the animals, and the limited times selected for postsurgical evaluation. Although our morphometric data appeared to approach statistical significance with our sample size and increased numbers might become statistically significant, it would not change our conclusions as there were no meaningful functional deficits with animals. In addition, post hoc statistical analysis of EMG was found to be 16%, and future studies with increased numbers may detect a difference. We selected the two times owing to the short half-life of tranexamic acid and time of detectable nerve injury [16, 17, 27]. As our exposure might not entirely simulate the complex environment of a THA, there is concern for potential increased invasiveness of the procedure. As such, there is the possibility that with increased inflammation and blood flow, there might be increased drug availability to the nerve. However, as the supratherapeutic concentration in our animal model did not have an adverse effect, this should account for the possibility of increased absorption of tranexamic acid with an actual THA. As the inflammatory cascade takes time to develop, the peak inflammatory and vascular response is seen approximately 7 days after injury to nerve [38]. Because tranexamic acid has quick absorption (plasma concentration peak at 1 hour after intramuscular injection) and quick half-life, drug concentration will be gone before the peak inflammatory response develops [27]. Although we evaluated the most logical markers that might be affected by tranexamic acid on the sciatic nerve, we did not exhaust all possibilities and did not evaluate the effects of tranexamic acid on other structures, such as the muscles and joint implants. The inability to assess pain in the animal model also may have limited our assessment regarding the effect of tranexamic acid on nerve function. Despite good intentions, bias may have been introduced during surgical dissection, tranexamic acid application, or during histologic evaluation of each animal group.
本研究有一定的局限性,包括动物数量少、动物非随机化选取,以及术后时间点选择有限。整体数据随着样本量和数据的增加而接近或具有统计学差异,不会改变我们的结论,因为动物没有明确的神经功能损伤。肌电图事后效能检验结果为16%,未来的研究有可能会因为样本量的不同而出现差异。因为氨甲环酸半衰期短和可检测神经损伤时间短,所以选择两个时间点[16,17,27]。由于动物实验暴露不能完全模拟人THA的复杂环境,因此有可能增加手术的侵入性。因此,随着炎症和血流量的增加,作用于坐骨神经的氨甲环酸量也会增加。然而,由于本文动物模型中超治疗浓度未见有副作用,这应该可以解释实际THA增加氨甲环酸吸收的可能性。由于炎症级联反应需要时间,神经损伤后7天左右可看到炎症和血管反应达峰值[38]。由于氨甲环酸具有快速吸收(血浆浓度在肌肉注射后1小时达到峰值)和快速半衰期,因此药物浓度将在峰值炎症反应出现之前消失[27]。虽然本文评估了可能受氨甲环酸影响的坐骨神经的最合理的标记物,但并没有用尽所有可能的方法,也没有评估氨甲环酸对其他结构的影响,例如肌肉和关节植入物。无法评估动物模型的疼痛也可能限制了氨甲环酸对神经功能影响的研究。尽管意图良好,但在外科解剖、氨甲环酸应用或每个动物组的组织学评估过程中可能会存在偏倚。
In the rat model, we found that electrophysiologic parameters, including nerve conduction velocity and amplitude of the sciatic nerve, did not differ from those after application of tranexamic acid compared with saline controls at 4 days and at 1 month after surgery, with the numbers available. Distal latency, however, was slightly increased at 4 days but not at 1 month (Table 1), which may have been secondary to early neural irritation and which resolved without any effects at 1 month. With acute and chronic nerve compression injuries, nerve conduction velocity is slowed [28]. Our results showed no functional effects to the sciatic nerve or hind-limb muscle activity after topical application of tranexamic acid compared with the control group at 1 month, with the numbers available. As tranexamic acid has a short half-life (2–3 hours) and has been shown to cause EMG changes during local application in the rat spinal cord [13,27], we hypothesized that tranexamic acid might have a transient effect or no effect on the sciatic nerve after local topical application. However, our results agree with our hypothesis. With one exception (distal latency in the 1mg tranexamic acid group at 4 days), with the numbers available, local application of tranexamic acid to the sciatic nerve had an effect on the parameters we studied in our model, which simulated surgical exposure of patients undergoing THA, at either 4 days or 1 month after application. Our findings likely reflect the selective effect of tranexamic acid on gamma-aminobutyric acid (GABA) receptors, which are located in the central nervous system, not in the peripheral nervous system (sciatic nerve) [13].
在大鼠模型中,根据现有数据,与生理盐水对照组相比,术后4天和1个月的电生理参数,包括坐骨神经的神经传导速度和波幅,与氨甲环酸实验组没有差异。然而,远端潜伏期在4天时略有增加,但1个月时没有增加(表1),这可能继发于早期神经刺激,并且在1个月时没有任何影响。急性和慢性神经压迫损伤时,神经传导速度减慢[28]。本研究结果显示,与对照组相比,1个月时,局部应用氨甲环酸对坐骨神经或后肢肌肉活动没有功能性影响。由于氨甲环酸的半衰期很短(2-3小时),并且已经证明在大鼠脊髓局部应用时会引起肌电图变化[13,27],我们假设氨甲环酸在局部应用后可能会对坐骨神经产生短暂影响或没有影响。然而,我们的结果与我们的假设一致。坐骨神经局部应用氨甲环酸对本文模型中的研究参数有一定影响,就是1 mg氨甲环酸组在4天时的远端潜伏期略有增加。该模型模拟患者接受THA手术时应用氨甲环酸4天或1个月两个时间点。本文结果可能反映了氨甲环酸对伽马氨基丁酸(GABA)受体的选择性作用,GABA受体位于中枢神经系统,而不在周围神经系统(坐骨神经)[13]。
We found no difference in macrophage recruitment at 4 days and at 1 month in tranexamic acid-treated sciatic nerve sections relative to controls, with the numbers available. This result differed from findings in a previous study in the rat [17], which showed that macrophage recruitment increased immediately in nerve sections that were acutely crushed compared with a gradual increase with long-term (months) chronic nerve compression. With trauma or surgery, injury to vascular endothelium results in exposure of collagen and release of tissue factors. The tissue factors and exposed collagen activate the extrinsic and intrinsic coagulation cascade, and in turn, plasminogen and the anticoagulation pathway. By competitively inhibiting the conversion of plasminogen to plasmin, tranexamic acid promotes the coagulation process. As tranexamic acid prevents degradation of clot formation, it is possible there is increased macrophage recruitment and analteration of the blood-nerve barrier [17, 24]. Therefore, in our study, we found no change in macrophage recruitment to suggest an inflammatory process involving the sciatic nerve when treated with topical application of tranexamic acid.
根据现有数据,氨甲环酸处理组坐骨神经切片在4天和1个月时巨噬细胞募集与对照组没有统计学差异。这一结果与之前大鼠的研究结果有所不同[17],该研究表明,与渐进性长期(数月)慢性神经压迫相比,急性挤压的神经切片中的巨噬细胞募集立即增加。创伤或手术使血管内皮损伤,导致胶原暴露和组织因子释放。组织因子和暴露胶原激活外源性和内源性凝血级联反应,进而激活纤溶酶原和抗凝途径。氨甲环酸通过竞争性抑制纤溶酶原向纤溶酶的转化,促进凝血过程。由于氨甲环酸可防止血栓降解,因此有可能增加巨噬细胞募集和血神经屏障的改变[17,24]。在本研究中,巨噬细胞募集没有变化,表明局部应用氨甲环酸时,炎症过程涉及坐骨神经。
When examining blood-nerve barrier integrity, our results also suggest that there is no 4-day or 1-month difference in the extravasation of Evans Blue albumin from the control nerve samples compared with the nerves treated with topical tranexamic acid, with the numbers available. In an animal study by Gray et al. [16], Evans Blue albumin extravasation increased in a sciatic nerve section at 2 and 4 weeks after compression injury. Omura et al. [25], in their study of acute nerve injury, reported that Evans Blue albumin leakage to the endoneurium was seen at 24 hours, peaking 3 to 7 days after injury, and returning to nearly normal levels at 21 days after injury. Similarly, Olsson [24] reported increased extravasation of Evans Blue albumin in an acutely crushed sciatic nerve rat model. Our results indicate that the blood-nerve barrier remains intact at 4 days and 1 month after topical administration of tranexamic acid, at which times an effect would be evident if there was to be one [16, 24, 25].
检测血-神经屏障完整性时,本研究结果表明,伊文思蓝白蛋白的外渗对照组与氨甲环酸实验组,术后4天或1个月时无统计学差异。Gray等[16]的一项动物研究表明,压迫损伤后2周和4周,坐骨神经伊文思蓝白蛋白外渗增加。Omura等[25]对急性神经损伤的研究中报告,损伤后24小时,伊文思蓝白蛋白泄漏到神经内膜,损伤后3至7天达峰值,并在损伤后21天恢复到接近正常水平。类似地,Olsson[24]报告急性挤压伤坐骨神经大鼠模型中伊文思蓝白蛋白的外渗增加。本研究结果表明,局部施用氨甲环酸后4天和1个月,血神经屏障保持完整,在这种情况下,如果存在伊文思蓝白蛋白外渗,那么外渗肯定很明显[16,24,25]。
Topical application of tranexamic acid has shown good results in decreasing blood loss in patients during THA. Further randomized controlled studies are needed to find the optimal application dose, along with the timing and frequency of administration [15, 20]. Our study suggests that local topical administration of tranexamic acid does not affect sciatic nerve function in an in vivo animal model, with the numbers available. However, because our statistical power was low, these data should be considered hypothesis-generating pilot data for larger, more-definitive studies. Our findings may help support the safety of local topical use of tranexamic acid during THA.
局部应用氨甲环酸可明显减少THA手术出血。需要进一步的随机对照研究,以找到最佳应用剂量,给药时间和频次[15,20]。本研究表明,根据现有数据,局部应用氨甲环酸不会影响动物模型体内坐骨神经功能。然而,由于我们的统计效能较低,本实验可做为验证假设的预实验,需要进一步更大规模、更明确的研究。本发现可能有助于支持THA期间局部安全使用氨甲环酸。
参考文献:略
,免责声明:本文仅代表文章作者的个人观点,与本站无关。其原创性、真实性以及文中陈述文字和内容未经本站证实,对本文以及其中全部或者部分内容文字的真实性、完整性和原创性本站不作任何保证或承诺,请读者仅作参考,并自行核实相关内容。文章投诉邮箱:anhduc.ph@yahoo.com






