有机合成路线中如何保护硝基(有机合成中引入硝基的方法有多少)
1.前言
硝基化合物,特别是芳香族硝基化合物,是具有悠久历史的一类化合物。硝基化合物是合成染料,香料,药物,和炸药的重要中间体,广泛应用于化学工业,制药和有机合成的各个领域中。因此,硝基化合物的制备是研究地最多,应用也最为广泛的一类有机反应。
同时,由于硝化,特别是芳香类化合物的硝化,需要过量的硝酸及硫酸等强酸,产生大量的废气和废酸,使之成为污染环境的一大因素。
硝基化合物的制备有多种途径,烃类的直接硝化,卤素取代及胺,肟的氧化等,如下图所示:

其中芳烃或脂肪烃的直接硝化是制备硝基化合物最简捷的途径。常见的硝化试剂归纳如下:
• 硝酸 Nitric acid
Alone or in combination with H2SO4, H3PO4, HClO4, HF(BF3), (CH3CO)2O, (CF3CO)2O, CF3CO2H, MeSO3H, CF3SO3H, FSO3H(SbF5), Nafion-H, sulfonated resins, clays, molecular sieves, graphite
• 硝酸盐 Nitrate salts
AgNO3/BF3, KNO3/H2SO4, K(Na)NO3/TMSCl/AlCl3, Cu(NO3)2/(CH3CO)2O, NH4NO3/(CF3CO)2O, (NH4)2Ce(NO3)
• 硝酸酯 Nitrate esters
BuONO2/Nafion-H, MeONO2/BF3, Me3SiONO2, acetone cyanohydrin nitrate
• 硝酰化合物 Nitryl compounds
NO2BF4, NO2PF6, NO2ClO4, NO2Cl(F), MeCO2NO2, CF3CO2NO2, PhCO2NO2, N-nitropyridinium nitrate
•氧化氮 Nitrogen oxides
NO2-O3, N2O3/BF3, N2O4/H2SO4, N2O4/AlCl3, nBuLi/N2O4, N2O5, N2O5/HNO3,N2O5/SO2
• 硝烷 Nitroalkanes
C(NO2)4, CH(NO2)3, (O2N)3CC(NO2)
在有机合成中,通过硝基化合物可以实现多种转换,如下图所示:
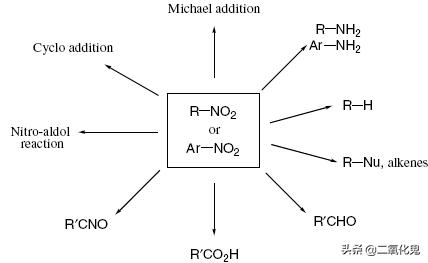
2.芳香族硝基化合物的合成
芳烃的直接硝化是合成芳香族硝基化合物最重要的方法。可使芳烃硝化的试剂很多,最常用的硝酸及其盐、以及硝酸酯、硝酸鎓盐、氧化氮类等。通常,硝化试剂的选择取决于硝化试剂及芳香族化合物的反应活性、硝化的区域选择性和一元硝化及多硝化的控制等因素。另外,重氮盐、硼酸被硝基取代,苯胺氧化,也能合成芳香族硝基化合物。
2.1 HNO3 作为硝化试剂反应示例
硝酸是最常用的硝化试剂之一,它能使许多芳香族化合物发生硝化,普通硝酸和发烟硝酸都能用于硝化。

A wide-mouth 1-L flask is supported inside a water bath. The flask is fitted with a moderate-speed stainless-steel propeller-type stirrer, and nitric acid (sp. gr. 1.4, 350 mL.) at 20° is poured into it. Veratraldehyde (70 g, 0.42 mole) is crushed at least as fine as rice grains and is slowly added in small portions to the acid. The rate of addition should be such that it requires about 1 hour to add all the aldehyde. The internal temperature is checked from time to time and should be held between 18° and 22°. The mixture is stirred for 10 minutes after the addition of the last of the aldehyde.
The mixture is then poured into 4 L of vigorously agitated cold water. From this point onward the protection of the product from light is extremely important. The stirring is continued for a few minutes; then the batch is filtered through Büchner funnel. The filter cake is recrystallized from 95% ethanol to give 55–60 g of pure material, melting at 132–133°. 1
2.2 HNO3 /H2SO4作为硝化试剂反应示例
硝酸与硫酸组成的混酸是比硝酸更强的硝化剂,根据底物活性的高低可以使用普通的硝酸或发烟硝酸,这个方法可以顺利地得到多硝基取代的产物。

A 5-L three-necked flask fitted with an all-glass addition funnel and two condensers is charged with 770 mL of concentrated sulfuric acid and 1.3 L of 90% fuming nitric acid. The solution is heated under gentle reflux, and a solution of (73 g, 0.4 mol) of 9-fluorenone in 840 mL of concentrated sulfuric acid is added from the dropping funnel over a 1-hour period. After the fluorenone addition is complete, a solution of 950 mL of fuming nitric acid in 1120 mL of concentrated sulfuric acid is added dropwise during 8.5 hours to the gently refluxing reaction mixture. The heating jacket is turned off and the solution is allowed to stand for 10 hours. The reaction mixture is poured into 5 gallons of water in two 5-gal Crocks. The light yellow precipitate is washed with water, twice by decantation, filtered, washed several times with water and sucked dry, and finally is dried in a vacuum oven at 80° for 10 hours. The yield of crude 2,4,5,7-tetranitrofluorenone, mp 249–253°, is 105–117 g (72–80%). This solid is recrystallized from 1.6 L of acetic acid containing 100 mL of acetic anhydride. The hot solution is filtered through a fluted filter and cooled rapidly to yield 80–86 g (51–54%) of 2,4,5,7-tetranitrofluorenone, mp 253.0–254.5°. 2
2.3 硝酸盐 /H2SO4作为硝化试剂反应示例
硝酸盐 /H2SO4是硝化常用的试剂,通常使用的硝酸盐是硝酸钾,反应条件温和,操作方便,反应迅速,通常在0oC进行,缺点是选择性不好。

4-bromo-2-tert-butylphenyl methyl carbonate (470 g, 1.67 mol) is dissolved in conc. H2SO4 (1000 mL) at 0 ℃. KNO3 (253 g, 2.5 mol) was added in portions over 90 minutes to the above solution and the reaction mixture is sequentially stirred for 2 hours at 0 ℃. The mixture is poured into 20 L ice-water. The resulting precipitate is collected and washed with water thoroughly, dried and recrystallized from ether to give the pure product (332 g, yield 60%)。
2.4 HNO3/Ac2O 作为硝化试剂反应示例
硝酸和醋酐混合,生成醋酸和硝酸的混酐(CH3COONO2),是一个良好的硝化试剂,反应条件比较温和。

A 1 L three-necked round-bottomed flask, fitted with a dropping funnel and a mechanical stirrer, is cooled in an ice-salt mixture. To the flask are added freshly distilled cinnamaldehyde (55.5 g, 50 mL, 0.42 mole) and of acetic anhydride (225 mL). When the temperature of the solution has reached 0–5°C, a solution of concentrated nitric acid (sp. gr. 1.42, 18 mL) in glacial acetic acid (50 mL) is added slowly through the dropping funnel while the mixture is stirred. The time of addition is 3–4 hours, during which the temperature is kept below 5°. After the addition is complete, the mixture is allowed to warm slowly to room temperature. The reaction flask is then dismantled and stoppered, and the reaction mixture is allowed to stand 2 days.
At the end of this time, hydrochloric acid (20%) is added cautiously to the cooled solution until a precipitate begins to appear. The addition of acid is then stopped, and the solution is allowed to cool in an ice bath or refrigerator until precipitation of the solid is completed. The light-yellow needles are collected on a Büchner funnel and dried in air. About 24 g of o-nitrocinnamaldehyde, mp 125–127°, is obtained. Additional product can be isolated by cautiously adding water to the mother liquor until precipitation is observed and then cooling the resultant mixture for several hours in an ice bath. Recrystallization from 95% ethanol gives 5–10 g. of o-nitrocinnamaldehyde, mp 126–127°. The total yield is 27–34 g (36–46%). 3
2.5 硝酸或硝酸铵/三氟乙酸酐作为硝化试剂反应示例
硝酸和三氟乙酸酐也是一个类似的硝化试剂,硝酸也可用硝酸铵代替。

A mixture of trifluoroacetic anhydride (10 mL) and fuming nitric acid (2.4 mL) was chilled at –15ºC and after 1 h a solution of furan-2-carbaldehyde (0.96 g, 10 mmol) in trifluoroacetic anhydride (2 mL) was slowly added to the reaction mixture keeping the temperature at -15ºC. The reaction mixture was stirred at -15ºC for 2 h and then the solvents were removed and pyridine (2 mL) was added to the reaction mixture, stirred for 15 min. And then the solvent was again removed and the oily residue was poured in ice and extracted with diethyl ether. The crude product was then purified over a silica gel column to give the pure product (0.96 g, 68 %). 4

5-Methylisoxazole (8.31 g, 0.1 mol) was dissolved in trifluoroacatic anhydride (74 g, 0.35 mol= 50 mL, d = -1.487 g/mL), and ammonium nitrate (8.00 g, 0.100mol) was added in 0.5-g portions, keeping the reaction temperature between 25 and 30 "C. After complete addition, 'H NMR of a neat aliquot showed the mixture to contain 65% product and 35% starting isoxazole. Thus, another portion of ammonium nitrate (3.60 g, 0.045 mol) was added as above. After complete addition, 'H NMR analysis indicated that less than 5% of starting material remained. The mixture was poured onto ice water and extracted with chloroform (4 X 50 mL). The extracts were washed with water (250 mL), and this aqueous wash was extracted with chloroform (3 X 25 mL). The combined chloroform extracts were dried over magnesium sulfate and filtered, and the filtrate was concentrated giving a yellow oil [12.34 g (96%)], which was virtually pure as determined by 'H NMR. The oil was distilled to give the product (8.04 g, 63%) as a pale yellow liquid: bp 88-90 "C (18 Torr).5
未完待续
如果您喜欢这篇文章,记得点赞收藏评论转发一键四连哦!!!
参考文献:
1. Organic Syntheses, Coll. Vol. 4, p.735; Vol. 33, p.65.
2. Organic Syntheses, Coll. Vol. 5, p.1029; Vol. 42, p.95.
3. Organic Syntheses, Coll. Vol. 4, p.722; Vol. 33, p.60.
4. Katritzky, A.R; Scriven, E. F. V; Majumder, S. ARKIVOC, 2005, 179.
5. L.A.Reiter, J.org.Chem. 1987, 52, 2714.
,免责声明:本文仅代表文章作者的个人观点,与本站无关。其原创性、真实性以及文中陈述文字和内容未经本站证实,对本文以及其中全部或者部分内容文字的真实性、完整性和原创性本站不作任何保证或承诺,请读者仅作参考,并自行核实相关内容。文章投诉邮箱:anhduc.ph@yahoo.com






